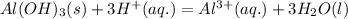Answer:
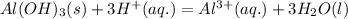
Step-by-step explanation:
- Net ionic equations are written by omitting same ions present in both side of total ionic equation.
- HBr and
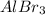 are strong electrolytes. Therefore they are fully dissociated in aqueous solution.
are strong electrolytes. Therefore they are fully dissociated in aqueous solution. - Total ionic equation:
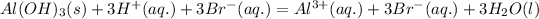
- Net ionic equation (by omitting
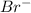 from both side):
from both side):