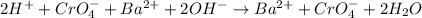Answer: Option (d) is the correct answer.
Step-by-step explanation:
An equation in which electrolytes are represented in the form of ions is known as an ionic equation.
Strong electrolytes easily dissociate into their corresponding ions. Hence, they form ionic equation.
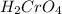 is a strong acid and
is a strong acid and
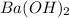 is a strong bases, therefore, both of them will dissociate into ions.
is a strong bases, therefore, both of them will dissociate into ions.
Thus, total ionic equation will be as follows.