Step-by-step explanation:
The given data is as follows.
Volume = 0.25 ml =
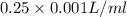 = 0.00025 l
= 0.00025 l
mass =
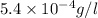
Also, it is known that molar mass of stearic acid is 284.48 g/mol.
Therefore, calculate the mass present in 0.00025 l as follows.
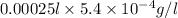
=
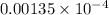
= 13.5 g
As number of moles is mass divided by molar mass of the substance. Hence, calculate the number of moles of stearic acid as follows.
No. of moles =
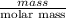
=
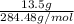
= 0.047 mol
Thus, we can conclude that there are 0.047 mol of steric acid are contained in the given solution.