Answer:The correct answer option A.
Step-by-step explanation:

Q= heat removed from the water
m= mass of the water = 80 grams
c = heat capacity of water= 4.18 J/g°C
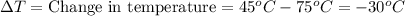
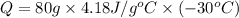
Q = -10,032 joules
 -10,000 Joules
-10,000 Joules
Negative sign indicates that heat was removed from the water.
Heat removed from the water of 80 gram of water is 10,032 Joules.Hence, the correct answer option A.