Answer:

Step-by-step explanation:
Molarity is found by dividing the moles of solute by liters of solution.
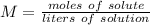
We know the molarity is 0.45 M and there are 0.750 liters of solution. The solute is NaCl (sodium chloride). We can substitute the values into the formula.
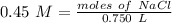
The molarity (M) can also be represented by mol/L
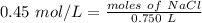
We are solving for the moles of solute, so we must isolate the numerator. It is being divided by 0.750 liters. The inverse operation is multiplication, so multiply both sides of the equation by 0.750 L.
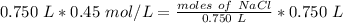
The liters will cancel out.
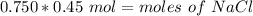
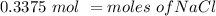
There are 0.3375 moles of NaCl in a 0.750 liter solution with a molarity of 0.45 M.