The two-step mechanism is:
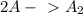
slow
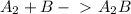
fast
The overall reaction is the sum of the two reactions:
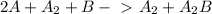
Now you can cancel the the terms that appear in both sideds:
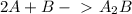
The fact that one reaction and the other is fast does not change the product. It does change the spped (rate) of reaction, but that is a different point.