Answer:
297.4332 grams of chlorine is present in 465 grams of calcium chloride.
Step-by-step explanation:
Mass of calcium chloride = 465 g
Molar mass of calcium chloride = 111 g/mol
Moles of calcium chloride =
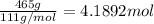
1 mol of calcium chloride has 2 moles of chlorine atoms.
Then 4.1892 moles of calcium chloride has:
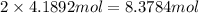 of chlorine atoms
of chlorine atoms
Mass of 8.3784 moles of chlorine atom:
8.3784 mol × 35.5 g/mol=297.4332 g