Answer:
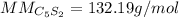
Step-by-step explanation:
Hello!
In this case, since the computation of the molar mass requires the molecular formula, we first realize that of pentacarbon disulfide is C₅S₂ due to the given prefixes. In such a way, we multiply the atomic mass of carbon by five and that of sulfur by two as shown below:
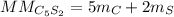
Thus, we plug in the atomic masses given in the periodic table to obtain:

Best regards!