Explanation:
a) We know that triangle ABD is a right triangle, because of the right angle mark show, and the fact that BD- height, thus perpendicular.
We also know that m<A=30 degrees, due to given diagram.
The sum of all angles in a triangle is 180 degrees. Therefore, m<BDA + m<ABD + m<DAB (Usain Bolt!) = 180 degrees.
m<BAD=30 degrees, m<BDA=90 degrees
Substitute these two in the equation above. You will get that m<ABD=60 degrees.
b) Triangle ABD is a special triangle, with angles of 30,60 and 90.
We know that AD=10
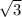 units.
units.
AD is opposite the 60 degree angle, or <ABD. It is a theorem that the angle opposite to the 60 degree angle is the height (BD) divided by
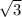 . Calculate, and what do you get? BD=10 units!
. Calculate, and what do you get? BD=10 units!
c) Use the fact that triangle ABC is an isosceles triangle.
d) Use the fact that you know the height, base, and triangle area formula: Base x 1/2 x Height.
I will say no more, but I hope you understand the rest based on your logic.