If 1000 ml (1 L) of CH₃COOH contain 1.25 mol
let 250 ml of CH₃COOH contain x
⇒ x =
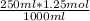
= 0.3125 mol
∴ moles of CH₃COOH in 250ml is 0.3125 mol
Now, Mass = mole × molar mass
= 0.3125 mol × [(12 × 2)+(16 × 2)+(1 × 4)] g/mol
= 18.75 g
∴ Mass of CH₃COOH present in a 250 mL cup of 1.25 mol/L solution of vinegar is
18.75 g