Answer: The particles that are emitted are alpha -particles.
Explanation: There are 3 types of particles that can be emitted in the radioactive decay process.
1) Alpha-particles: These particles are released in the reactions which undergo alpha-decay processes. The mass number of these particles is 4 and hence, the atom undergoing this decay will experience a change in its atomic mass.
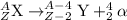
2) Beta-particles: These particles are released in the reactions which undergo beta-minus process. The mass number of these particles is 0 and hence, the atom undergoing this decay will not experience a change in its atomic mass.
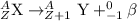
3) Positron particles: These particles are released in the reactions which undergo beta-plus processes. The mass number of these particles is 0 and hence, the atom undergoing this decay will not experience a change in its atomic mass.
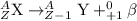
Hence, is the large atom decays, only alpha particle will change the atomic mass of that atom.