Answer:
The weight of Strontium-90 will reduce to
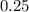 Kg in
Kg in
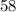 years.
years.
Step-by-step explanation:
Given -
Radioactive isotope sample of Strontium-90
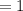 Kg
Kg
Half life of Strontium-90
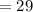 years
years
After first half life (i.e 29 years), the weight of Strontium-90
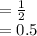 Kg
Kg
After second half life (i.e 58 years), the weight of Strontium-90
 Kg
Kg
Thus, the weight of Strontium-90 will reduce to
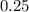 Kg in
Kg in
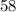 years.
years.