Answer:
Molarity
Step-by-step explanation:
There are many ways of measuring the concentration of a solution. Molarity is one of them.
Molarity is defined as the number of moles present in one liter of the solution. It is basically the ratio of the moles of the solute to the liters of the solution.
The expression for the molarity, according to its definition is shown below as:
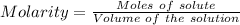
Where, Volume must be in Liter.
It is denoted by M.