Answer : The percent yield of the reaction is, 87.0 %
Solution : Given,
Mass of
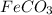 = 75.0 g
= 75.0 g
Molar mass of
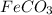 = 115.85 g/mole
= 115.85 g/mole
Molar mass of
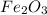 = 159.69 g/mole
= 159.69 g/mole
First we have to calculate the moles of
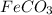 .
.
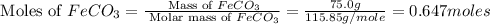
Now we have to calculate the moles of
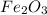
The balanced chemical reaction is,
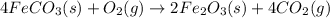
From the balanced reaction we conclude that
As, 4 mole of
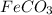 react to give 2 mole of
react to give 2 mole of
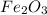
So, 0.647 moles of
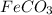 react to give
react to give
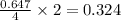 moles of
moles of
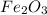
Now we have to calculate the mass of
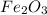
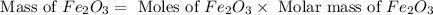
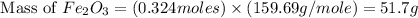
Theoretical yield of
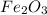 = 51.7 g
= 51.7 g
Experimental yield of
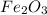 = 45.0 g
= 45.0 g
Now we have to calculate the percent yield of the reaction.
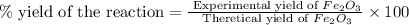
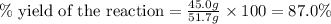
Therefore, the percent yield of the reaction is, 87.0 %