Answer: correct option will D.
Step-by-step explanation:
Calcium belongs to the group 2 i.e. of alkaline earth metals in periodic table. electronic configuration of calcium is
![[Ar]4s^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/scdzae3rdn8hrul4o3iyupeyr05olgmuo8.png) .
.
So in order to attain noble gas configuration calcium will loose its two electrons during chemical bond formation.
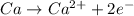
Sulfur is a non metal which forms negatively charged ion so as to complete ts octet during chemical bond formation.
Helium is a noble gas with fully filled electronic configuration.
Carbon forms bond which is covalent in nature.