Answer:
26.314KJ
Step-by-step explanation:
The specific heat capacity formula is :
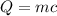 Δ
Δ
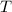 , where Q is the heat energy, m is the mass, c is the heat capacity of the material, and delta T is the change in temperature.
, where Q is the heat energy, m is the mass, c is the heat capacity of the material, and delta T is the change in temperature.
We know from the question that m = 386grams and delta T = 100.0°C - 24.0°C = 76.0°C. From a simple search, we know the specific heat capacity of aluminum = 0.897J/g°C (this changes a little bit depends on the website you use, but the value should be very similar). Let's apply the formula:
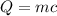 Δ
Δ
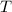
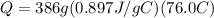
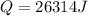 or
or
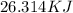
Round according to your teacher's specification (example: significant figures) if needed.