Answer: The mass of solution of
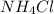 is 4.86 grams.
is 4.86 grams.
Explanation:
In
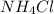 , there are 1 nitrogen atom , 4 hydrogen atoms. and 1 chlorine atom.
, there are 1 nitrogen atom , 4 hydrogen atoms. and 1 chlorine atom.
To calculate the mass percent of element in a given compound, we use the formula:
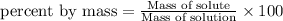
Percent by mass of solution = 35%
Mass of solute
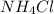 = 1.7 gram
= 1.7 gram
Mass of solution = ?
Putting values in above equation, we get:
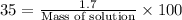
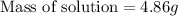
Hence, the mass of solution is 4.86 grams.