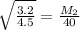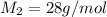Answer: c) 28 g/mol
Explanation: Rate of diffusion : It is defined as the volume of gas effused in a given time 't'.
Formula used :
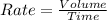
According to Graham's law of diffusion, rate of diffusion is inversely proportional to the square root of the mass of the gas.
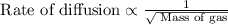
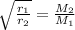
where,
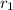 = rate of diffusion of argon gas = 3.2cm/sec
= rate of diffusion of argon gas = 3.2cm/sec
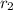 = rate of diffusion of unknown gas = 4.5 cm/sec
= rate of diffusion of unknown gas = 4.5 cm/sec
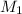 = Molar mass of argon gas= 40g/mol
= Molar mass of argon gas= 40g/mol
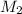 = Molar mass of unknown gas
= Molar mass of unknown gas