Answer:
1218.672g (1220 g with sig figs)
Step-by-step explanation:
molar highway: g <-molar mass-> mol <-avogadro-> particle
right now you are at moles, and you have to move to grams by using the molar mas
atomic mass of carbon = 12.01 amu x 6 = 72.06
atomic mass of hydrogen = 1.01 amu x 6 = 6.06
78.12 = molar mass of C6H6
set up the conversions:
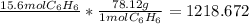
take sig figs into account and it rounds to 1220 grams