Answer: The correct option is' gas < liquid < solid '.
Step-by-step explanation:
Density is defined as mass of substance present in a unit volume of the substance. it is measured in kilogram per meter cube,
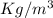 .
.
Density in states of matter:
- In solids, molecules are tightly packed and the movement of molecules are also restricted. Since molecules strongly associated with each other, more amount of mass will be present in a unit volume.
- In liquids, molecules are weakly associated with each other and movement of molecules are not restricted. Since molecules are weakly associated, less mass will be present in a unit volume.
- In gases, molecules are loosely packed that is they are very weakly associated with each other and movement of molecules are not restricted. Since, the molecules are loosely packed, lesser amount of mass will be present in a unit volume.
So, the order from least dense to most dense state is:
gas < liquid < solid