Answer:
The volume occupied by the air inside the hot balloon is 2.67 L.
Step-by-step explanation:
Initial volume of the balloon =
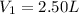
Initial temperature of the air inside the balloon
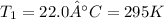
Final volume of the balloon =
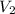
Final temperature of the air inside the balloon =
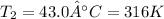
Using Charles law:
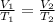
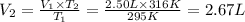
The volume occupied by the air inside the hot balloon is 2.67 L.