Step-by-step explanation:
Iron is a transition element and its atomic number is 26 with electronic distribution
![[Ar]4s^(2)3d^(6)](https://img.qammunity.org/2018/formulas/chemistry/high-school/8qiir60m7lyohjv1qt0q17c1g1x8rcxsc3.png) .
.
So, 4s shell being larger in size as compared to 3d shell so, when two electrons are lost then they will be lost from 4s shell first.
Hence, electronic distribution will then become
![[Ar]4s^(0)3d^(6)](https://img.qammunity.org/2018/formulas/chemistry/high-school/nyn3nvq28zfrazlc4dbnil3ufa3viwha23.png) .
.
As a result,
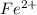 will be formed and name of this ions is ferrous ions.
will be formed and name of this ions is ferrous ions.