Step-by-step explanation:
1) Answer 2:
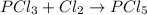
According to reaction, 1 mol of chlorine gas gives 1 mol of
![PCl_5]](https://img.qammunity.org/2018/formulas/chemistry/high-school/7u7vkek340zyyixd4r2n4miy3nzbb5d4ol.png)
Then, 3.00 moles of chlorine gas will give :
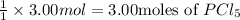
3.00 moles of
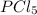 are made.
are made.
2) Answer 3:
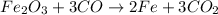
Molar ratio is defined ratio of moles of two different substances.
Moles of carbon monoxide gas = 3 mol
Moles of carbon dioxide gas = 3 mol
Molar ratio of
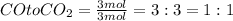
Molar ratio of
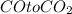 is 3:3.
is 3:3.
3) Answer 4:
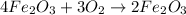
Moles of oxygen gas =
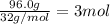
According to reaction 3 moles of oxygen gas reacts with 4 mol of iron metal.
Then mass of 4 mol of iron metal :4 mol × 55.85 g/mol =223.4 g≈223 g
223 grams of iron was oxidized.
4) Answer 5;
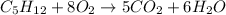
According to reaction , 5 moles of carbon dioxide are produced by 1 mol of pentane.
Then 10 moles of carbon dioxide will be produced by:
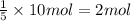 of pentane.
of pentane.
2 moles of pentane would need to be supplied.