Answer:
2.06×
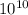 atoms
atoms
Step-by-step explanation:
The radius of potassium atoms= 231 pm
convert parts per million into m i.e, by multiplying by
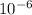
= 231×
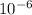
Potassium atoms would have to laid down at a distance apart= 4.77 mm
convert millimeter into meter i.e, by multiplying by
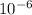
= 4.77 ×
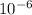
Now, we have to find the number of potassium atoms,
Number of potassium atoms=
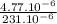
Number of potassium atoms=2.06×
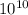
Number of potassium atoms= 2.06×
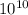 atoms
atoms
Hence, the correct answer is 2.06×
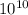 atoms
atoms