Answer: Chlorine (Cl) is the oxidizing agent because it gains an electron.
Step-by-step explanation:
The redox reaction is:
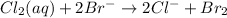
On reactant side:
Oxidation state of chlorine = 0
Oxidation state of bromine = -1
On product side:
Oxidation state of chlorine = -1
Oxidation state of bromine = 0
The oxidation state of chlorine reduces from 0 to -1 it is getting reduced by gain of electrons and it undergoes reduction reaction. As chlorine gets reduced, it acts as oxidizing agent.
The oxidation state of bromine increases from -1 to 0. Thus, it is getting oxidized by loss of electrons and it undergoes oxidation reaction.As bromine gets oxidized, it acts as reducing agent.
Thus Chlorine (Cl) is the oxidizing agent because it gains an electron.