Answer : The correct option is, Silicon (Si)
Explanation :
Element = Helium
Atomic number = Number of electrons = 2
The electronic configuration will be,
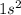
Element = Barium
Atomic number = Number of electrons = 56
The electronic configuration will be,
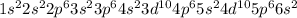
Element = Nitrogen
Atomic number = Number of electrons = 7
The electronic configuration will be,
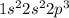
Element = Silicon
Atomic number = Number of electrons = 14
The electronic configuration will be,
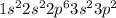
Element = Sulfur
Atomic number = Number of electrons = 16
The electronic configuration will be,
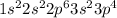
From the electronic configurations, we conclude that the element silicon has two electrons in its valence 'p' orbital or outermost 'p' orbitals.
Hence, the correct option is, Silicon (Si)