Answer:
The new volume of the ballon will be 13.345 L
Step-by-step explanation:
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure, by means of a constant of proportionality that is applied directly. For a given sum of gas at a constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases.
Charles's law is a law that says that when the amount of gas and pressure are kept constant, the quotient that exists between the volume and the temperature will always have the same value:
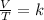
It is possible to assume that you have a certain volume of gas V1 that is at a temperature T1 at the beginning of the experiment. If you vary the volume of gas to a new value V2, then the temperature will change to T2, and it will be true:
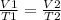
In this case:
- V1= 17 L
- T1= 200 K
- V2= ?
- T2= 157 K
Replacing:
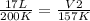
Solving:
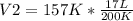
V2= 13.345 L
The new volume of the ballon will be 13.345 L