Answer:
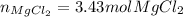
Step-by-step explanation:
Hello!
In this case, since the reaction between magnesium and hydrochloric acid is:
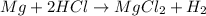
Whereas there is a 1:1 and 1:2 mole ratio between magnesium and magnesium chloride and hydrochloric acid and magnesium chloride respectively; it is possible to compute the yielded moles of product by each reactant and then determine which moles are correct based on the limiting reactant; therefore, we proceed as follows:
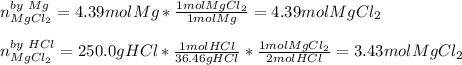
Thus, since the hydrochloric acid yields the fewest moles of product, we infer it is the limiting reactant, so the correct number moles of magnesium chloride are 3.43 mol.
Best regards!