Answer: 94.59 grams of 92.5 %
 by mass solution will be needed.
by mass solution will be needed.
Step-by-step explanation:
Mass of sulfuric acid is 250 grams of 35 % by mass solution:
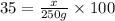
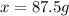
Mass of
 in 250 g of 35 % solution = 87.5 g
in 250 g of 35 % solution = 87.5 g
Mass of 92.5 %
 needed to make 35 % by mass solution.
needed to make 35 % by mass solution.
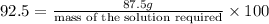
Mass of the solution required = 94.59 g
94.59 grams of 92.5 %
 by mass solution will be needed.
by mass solution will be needed.