Answer:
D) It is not spontaneous at any temperature.
Step-by-step explanation:
Given reaction:
CaSO4(s) + 2HCl(g) → CaCl2(s) + H2SO4(l)
ΔH = +8.91*10³ J
ΔS = -219.20 J/K
The sign of the Gibbs free energy (ΔG) determines the spontaneity of a given reaction. ΔG is related to ΔH and ΔS as follows:
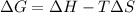
A reaction is spontaneous only when ΔG is negative i.e. ΔG < 0
Under the given conditions:
ΔH > 0 ; ΔS <0 and ΔH > ΔS
i.e. ΔG = ('+' value) - T( '- 'value) = + value
Therefore, irrespective of the temperature, ΔG will always be positive