Answer:
35.34 atoms will be present after 14,325 years.
Explanation:
Given : Carbon-14 has a half-life of approximately 5,730 years. This exponential decay can be modeled with the function N(t) = N0. If an organism had 200 atoms of carbon-14 at death.
To find : How many atoms will be present after 14,325 years?
Solution :
The half-life exponential function modeled is
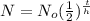
Where,
 is the initial atoms
is the initial atoms
N is the total number of atoms.
t=14,325 years is the time
h=5,730 years is the half-life time
Substitute the value in the formula,
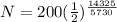
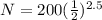
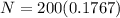
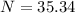
Therefore, 35.34 atoms will be present after 14,325 years.