Answer:
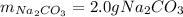
Step-by-step explanation:
Hello,
In this case, we consider the equation defining molarity, in order to compute the mass that is present into 125 mL of an aqueous 0.15M of sodium carbonate (solute), Na₂CO₃ as shown below:
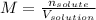
Now, since the unknown is the mass which comes from the moles, by solving for it and subsequently using its molar mass, one obtains:
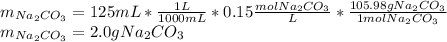
Best regards.