Answer:
6.07 g.
Step-by-step explanation:
Hello!
In this case, given the chemical reaction:
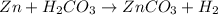
We first need to compute the moles of each reactant considering their molar masses:
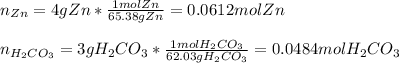
Now, compute the mass of zinc carbonate yielded by each reactant in order to pick out the correct limiting reactant:
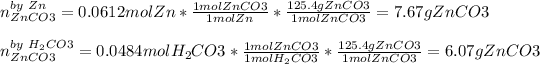
Thus, we conclude carbonic acid is the limiting reactant as it produces the fewest grams of product and therefore the yielded mass of zinc carbonate product 6.07 g.
Best regards.