Answer:
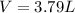
Step-by-step explanation:
Hello,
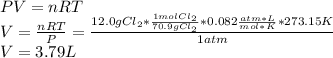
In this case, we are talking about an ideal gas problem in which 12.0 g of chlorine gas is at standard both pressure (1atm) and temperature (0°C), therefore, to compute the corresponding volume, one applies the ideal gas law, then converts from grams to moles considering the diatomic chlorine and subsequently solves for volume as shown below:
Best regards.