Answer: 3.2
Step-by-step explanation:
pH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/college/kv1d6x7k5u8rrmnok6mdbtm4oardzajzce.png)
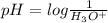
Thus as pH and
 are inversely related, a solution having lower pH will have more amount of
are inversely related, a solution having lower pH will have more amount of
 concentration. And a solution having more pH will have less amount of
concentration. And a solution having more pH will have less amount of
 concentration.
concentration.
Thus the solution with lowest pH of 3.2 will have highest hydronium ion concentration.