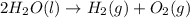Answer:
Explanation :
Electrolysis of water is defined as decomposition of water into oxygen gas and hydrogen gas due to the passage on an electric current. In electrolytic cell oxidation occurs at anode and reduction occurs at cathode. At anode we will get oxygen gas and at cathode we will get hydrogen gas.
Half reaction at anode:
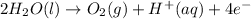 ..(1)
..(1)
Half reaction at cathode:
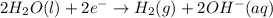 ..(2)
..(2)
Overall reaction:(1)+(2)