Gay-Lussac's law gives the relationship between pressure and temperature of gas. For a fixed amount of gas, pressure is directly proportional to temperature at constant volume.
P/T = k
where P - pressure , T - temperature and k - constant
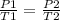
parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
substituting the values in the equation
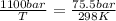
T = 4342 K
initial temperature was 4342 K