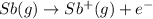Answer: The reaction for the first ionization of Sb is
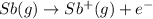
Explanation: Ionization of an atom is defined as the reaction when an electron is released from an isolated gaseous atom in their gaseous state.
General equation for the first ionization reaction is:
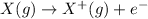
So, from the given choices in the question, only one option represents the first ionization of Antimony atom (Sb-atom), which is