Answer:
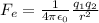
Step-by-step explanation:
The electrostatic force (also called Couloumb force) between two electrical charge is given by the following formula:
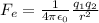
where:
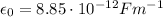 is the vacuum permittivity
is the vacuum permittivity
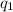 is the magnitude of the first charge
is the magnitude of the first charge
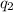 is the magnitude of the second charge
is the magnitude of the second charge
 is the distance between the two charges
is the distance between the two charges
This formula gives only the magnitude of the force. The direction depends on the relative sign of the two charges:
- If the two charges have same sign, the force is repulsive (this corresponds to a positive sign in the formula)
- If the two charges have opposite sign, the force is attractive (this corresponds to a negative sign in the formula)