Answer: option D. Interference patterns formed by electrons
Explanation:
Light behaves like wave as well as a particle. Light shows wave phenomenons such interference and diffraction and particle nature as seen in photoelectric effect.
Similarly matter also behaves like both particle and wave. de Broglie wave is associated with the matter. It means, whenever a matter moves, a de Broglie wavelength can be associated with it.
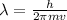
where,
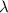 is the de broglie wavelength, h is the Planck's constant, m is the mass of the matter and v is the velocity. (mv) is the momentum of the matter.
is the de broglie wavelength, h is the Planck's constant, m is the mass of the matter and v is the velocity. (mv) is the momentum of the matter.
Matter waves were observed in an experiment with electrons which showed interference pattern same as wave phenomenon. Thus, option D is correct.