Answer:
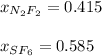
Step-by-step explanation:
Hello!
In this case, since the mole fraction of both gases in the tank is computed via:
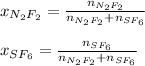
It means we need to compute the moles of each gas, just as it is shown down below:
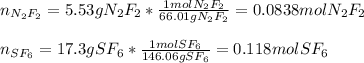
Thus, the mole fractions turn out:

Best regards!