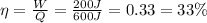The thermal efficiency of an engine is
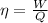
where
W is the work done by the engine
Q is the heat absorbed by the engine to do the work
In this problem, the work done by the engine is W=200 J, while the heat exhausted is Q=600 J, so the efficiency of the machine is