Answer:

Step-by-step explanation:
Hello!
In this case, for the separation of the benzene-toluene mixture, we can use the following mole balances including the given mole fraction at each stream per species:
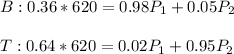
That can be solved by using a solver for P1 (benzene-rich flow) and P2 (toluene-rich flow):

Best regards!