Answer:
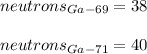
Step-by-step explanation:
Hello!
In this case, since isotopes of the same element have the same number of protons and electrons but different atomic mass, we can compute the number of neutrons by subtracting the number of protons to the atomic mass of the isotope; thus, for Ga-69 and Ga-71 (rounded up to whole numbers), we obtain:
Best regards!