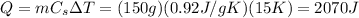The amount of heat needed to raise the temperature of a substance by

is given by
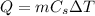
where
m is the mass of the substance
Cs is its specific heat capacity

is the increase in temperature
For oxygen, the specific heat capacity is approximately
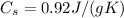
The variation of temperature for the sample in our problem is
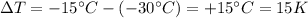
while the mass is m=150 g, so the amount of heat needed is