The given question is incomplete. The complete question is:
A chemist adds 35.0 mL of a 2.82M sodium nitrate (NaNO3) solution to a reaction flask. Calculate the millimoles of sodium nitrate the chemist has added to the reaction flask.
Answer: 98.7 mmol
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
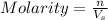
where,
n = milli moles of solute
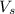 = volume of solution in ml
= volume of solution in ml
Now put all the given values in the formula of molarity, we get
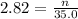
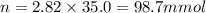
Therefore, the millimoles of sodium nitrate the chemist has added to reaction flask are 98.7