Answer:
Mass of MgO produced = 5.72 g
Step-by-step explanation:
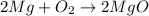
Mass of Mg = 3.45 g
Molar mass of Mg = 24.305 g/mol
No. of mol of Mg =
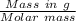
No. of mol of Mg =
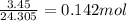
From the reaction coefficient,
2 mol of Mg form 2 mol of MgO
So, 0.142 mol of Mg form 0.142 mol of MgO
Molar mass of MgO = 40.3044 g/mol
Mass of MgO in g = No. of mol × Molar mass
= 0.142 mol × 40.3044 g/mol
= 5.72 g