Answer:
2Sb + 3I₂ → 2SbI₃
Step-by-step explanation:
The reaction expression we need to balance is:
Sb + I₂ → SbI₃
To balance this expression, we need to assign the variables a,b and c to each specie as the coefficient that will balance the expression.
aSb + bI₂ → cSbI₃
Conserving Sb : a = c
I : 2b = 3c
let a = 1, c = 1, b =
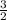
Multiply through by 2;
a = 2, b = 3 and c = 2
2Sb + 3I₂ → 2SbI₃