Answer:
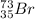
Step-by-step explanation: Atomic number is the number of protons or number of electrons.
Mass number is the sum of number of protons and number of neutrons.
Thus atomic number will be 35 and mass number is 35+38=73.
The representation of element is :
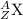
where Z= atomic number, A= mass number , X= chemical symbol of element
The representation of isotope of bromine with atomic number 35 and mass number 73 is :
