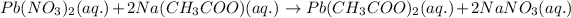Answer:
No precipitate forms in this reaction.
Step-by-step explanation:
It is an example of double displacement reaction.
In double displacement reaction, cations and anions of two salts interchange between themselves.
Here reaction between
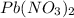 and
and
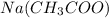 forms
forms
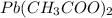 and
and
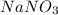 .
.
Both
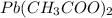 and
and
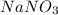 are soluble in water. Hence no real precipitate forms in this reaction.
are soluble in water. Hence no real precipitate forms in this reaction.
Balanced chemical equation: